07 January 2021
09 December 2020
pH as Abiotic Factor of environment
pH as abiotic factor
The term "pH" was first described by Danish
biochemist Søren Peter Lauritz Sørensen in 1909. pH is an
abbreviation for "power of hydrogen" where "p" is
short for the German word for power, potenz and H is the element symbol for
hydrogen.
pH is determined by the concentration of hydrogen
ions (H+ ). It is a measure of the acidity and alkalinity, on a scale from 0 to
14.
1.
Acidic solutions
have a pH less than 7,
2.
The basic or
alkaline solutions have a pH greater than 7.
3.
The pH 7
Indicate neutral. An example
of a neutral solution is pure water at room temperature.
pH
plays an important role in the survival of animals, including human beings? Our
body works well within a narrow pH range of 7 to 7.8. If, due to some reason,
this pH gets disturbed or change in the body of a person, then many ailments
can occur. The pH value of Hcl in stomach is between 1.5 to 3.5.
Pure
water has a pH of 7, which is neutral. Higher numbers indicate alkaline
water, the greater, the more alkaline. Lower numbers indicate acidity, the
lower the pH, the more acid.
The
ideal pH level of drinking water should be 6 to 8.5 the human body maintains pH
equilibrium on a constant basis and will not be affected by water consumption.
Measurement
of pH:
There
are multiple methods of measuring pH.
1. The most common method is a pH meter, which involves
a pH-sensitive electrode (usually made of glass) and a reference electrode.
2. Litmus paper is probably the most familiar pH paper.
It is used to test whether a solution is acidic or
basic or neutral and comes in following
three types—
a)
Red
b)
Blue,
c)
Neutral.
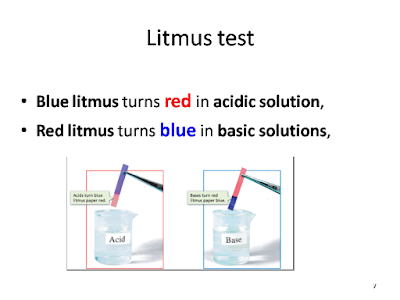
Red litmus turns blue in basic solutions,
Blue litmus turns red in acidic solution,
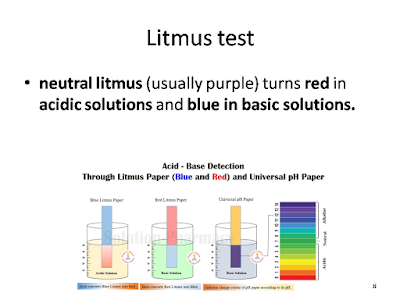
neutral litmus (usually purple) turns red in acidic solutions and blue in basic solutions.
3. A colorimeter may be used to measure the pH of a
sample.
Impact
of pH on Plants
pH
affects plant growth primarily through its effects on nutrient availability.
High or low pH cause deficiencies in essential nutrients that plants need to
grow.
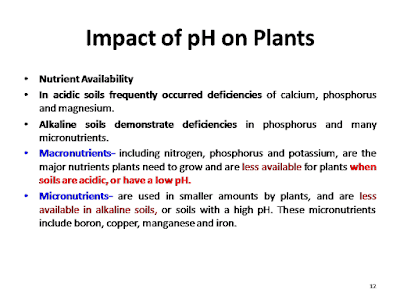
Nutrient
Availability
In
acidic soils frequently occurred deficiencies of calcium, phosphorus and magnesium.
Alkaline
soils demonstrate deficiencies
in phosphorus and many micronutrients.
Macronutrients,
including nitrogen, phosphorus and potassium, are the major nutrients plants
need to grow and are less available for plants when soils are acidic, or have a
low pH.
Micronutrients
are used in smaller amounts by plants, and are less available in alkaline
soils, or soils with a high pH. These micronutrients include boron, copper,
manganese and iron.
Impact
on Soil Microorganisms
The
microorganism such as bacteria and other organisms that inhabit the soil, are
most active at a pH of 6.3 to 6.8. These organism are helps in the processes of break down plant waste, such as
leaves, are most active at that level.
Some plants, such as blueberries and rhododendrons,
like acidic soils, while vegetables and most ornamentals thrive in slightly
acidic conditions. Plants such as lily and chrysanthemum are able to tolerate
slightly alkaline soils.
Nutrient
Availability
Many
of the elements most used by plants in their growth are less available when the
pH is in the acid range, below 6, and many of the micronutrients, those used in
small quantities, are less available when the water, or soil, is alkaline.
Many
common vegetables and ornamentals prefer soil with a pH of around 6.5 These
include spinach, parsnips, dahlias, chrysanthemums, sweet peas and tulips.
Chlorosis
One
common effect of the application of water with a high pH, or of a high soil pH,
is chlorosis, or the yellowing of the leaves in such a way that the leaf veins
remain green
Necrosis
in plant;
When a living organism's cells or tissues die or
degenerate, the condition is called necrosis.
Incorrect
soil pH for the plant can cause nutrient deficiency. Nutrients for which
deficiency leads to necrosis include potassium, nitrogen, boron, iron and
nickel.
Impact
of pH on animal
In
aquatic animals like fish can survive in lake or river water within a narrow
range of pH change.
Most
freshwater lakes, streams, and ponds have a natural pH in the range of 6 to
8. Acid deposition has many harmful ecological effects when the pH of most
aquatic systems falls below 6 and especially below 5.
Effect
on survival:
When
the pH of rain water is about 5.6 then it is acid rain. Too much
acidic rain makes difficult for the aquatic animals to survive and can even
kill the aquatic animals.
Effect
on animal distribution:
As
the pH of water body approaches 5, undesirable species of plankton and mosses
may begin to invade leads to decrease in populations of fishes even though they
disappear from the water body. Generally, below a pH of 4.5, the water is essentially
devoid of fish.
Effect
on respiration:
Aluminium
ions attached to minerals in nearby soil can be released into lakes, where they
can kill many kinds of fish by stimulating excessive mucus formation in gill,
and death of fish due to asphyxiation.
Effect
on reproduction:
The
most serious chronic effect of increased acidity in surface waters appears to
be interference with the fish’ reproductive cycle. It affect egg laying
capacity of female.
Impact
on Ammonia toxicity:
High pH may also increase the toxicity of
other substances. For example, the toxicity of ammonia is ten times more severe
at a pH of 8 than it is at pH 7.
It is directly toxic to aquatic life when it
appears in alkaline conditions. Low concentrations of ammonia are generally
permitted for discharge.
Acid
rain
Acid
rain can be caused by volcanic eruptions, but recently it has been attributed
to the burning of fossil fuels along with industrial byproducts being spewed
into the atmosphere.
1.
Damage to
Forests, Plants, and the Food Web
2.
Reduced pH Level
in Water.
3.
Poisoning of the
Soil
Ex.
Acid
rain leached calcium from the soil, which was the primary source of calcium for
snails in that environment.
The
snails soon died off, which was the primary source of calcium for birds in that
habitat. The birds had to look to other sources for their calcium, such as
insects. The birds were not able to receive a significant amount of calcium and
began to lay defective eggs.
Maintaining
pH
1. Natural processes such as rainfall lower pH by leaching
out minerals that make soils alkaline.
2. Fertilizers that contain ammonium, urea or
organic matter also tend to lower pH.
3. The most common additive used to raise pH is lime.
4. calcium carbonate is added to acidic lake water to
neutralise the acid that comes from
acid rain. This prevents the aquatic animals from being killed.
Parental care in fishes
Population interaction - Intra-specific Association Parental care in fishes INTRODUCTION • Looking after the eggs or young until they ...
-
Autecology and Synecology Ecological studies are based on three basic principles: H...
-
Unit 1: Introduction to Ecology Point to be Covered : 1. History of ecology 2. Autecology 3. Synecology E...
-
Aim: To Estimation of Dissolved oxygen from water sample All living organisms depend upon oxygen to maintain the metabolic processes th...